By Danae A. Black, Amanda Mummert, Amanda G. Althoff, and Lawrence Rasouliyan | OMNY Health
Tuberculosis (TB) has been a continuous public health challenge in the United States. Even though there are numerous screening and treatment options available, the burden of active TB disease still rises. Almost 80% of TB cases reactivate latent TB infection (LTBI). Timely identification of at-risk individuals and understanding their screening patterns is primarily important to reduce disparities in care.
Building the Study: From EHR Data to Insights
Our research team at OMNY Health conducted a retrospective, observational study using the OMNY Health Real-World Data Platform (2020–2024), which integrates electronic health records (EHRs) from multiple U.S. health systems. The goal was to describe individuals diagnosed with respiratory TB and evaluate patterns of TB screening and latent TB diagnosis before the onset of active TB disease.
Study Design and Methods
OMNY Health dataset identified the patients with respiratory TB (ICD-10-CM: A15). The earliest date of respiratory TB diagnosis was considered the index date. Demographic characteristics and social determinants of health (SDoH) were summarized at the index date or during the pre-index period.
Utilization of TB screening procedures (CPT: 86480, 86481, 86580; ICD-10-CM: Z11.1) or diagnosis of latent TB (ICD-10-CM: Z22.7, Z86.15) was evaluated during the pre-index period. Descriptive statistics were reported for all variables of interest.
Results: Identifying Patterns in Screening and Latent TB
Between the years 2020-2024, total 6,538 cases of respiratory TB were diagnosed. Among these cases, 238 had a latent TB diagnosis code before the index date and 20% showed evidence of TB screening prior to diagnosis.
TB testing was significantly higher among females and younger people, whereas it was lower among nonwhite and Hispanic groups. There had been more latent TB recorded among Hispanic individuals. This is consistent with high-risk profiles often seen among overseas-born populations or the ones travelling to counties where it is common.
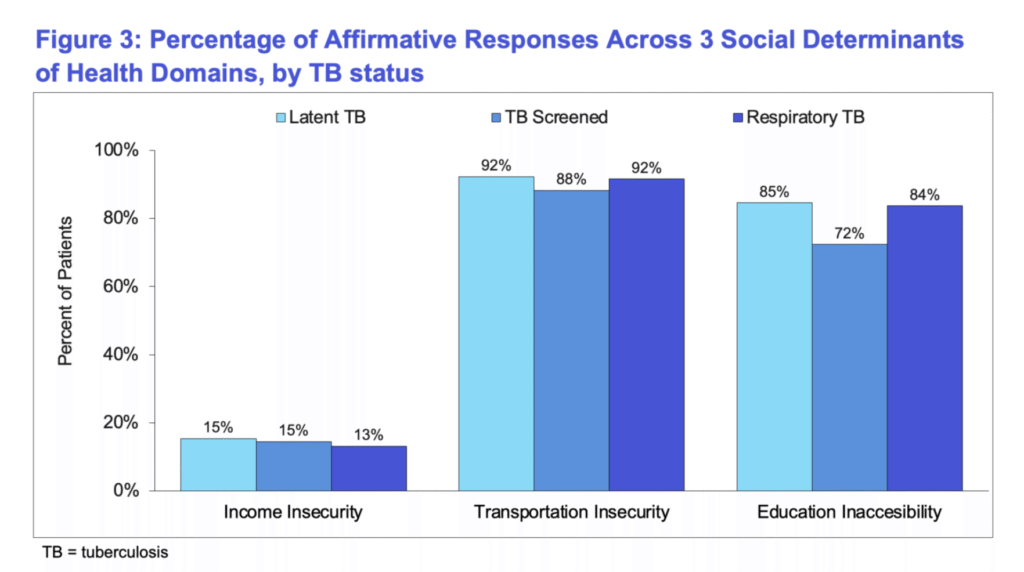
Approximately 5% of the population had data available on social health determinants, which revealed certain transportation and education barriers impeding prevention measures or treatment adherence.
Figure 1. TB Prevention Pathway
Figure 2. Study Population Demographics Characteristics, by TB Status
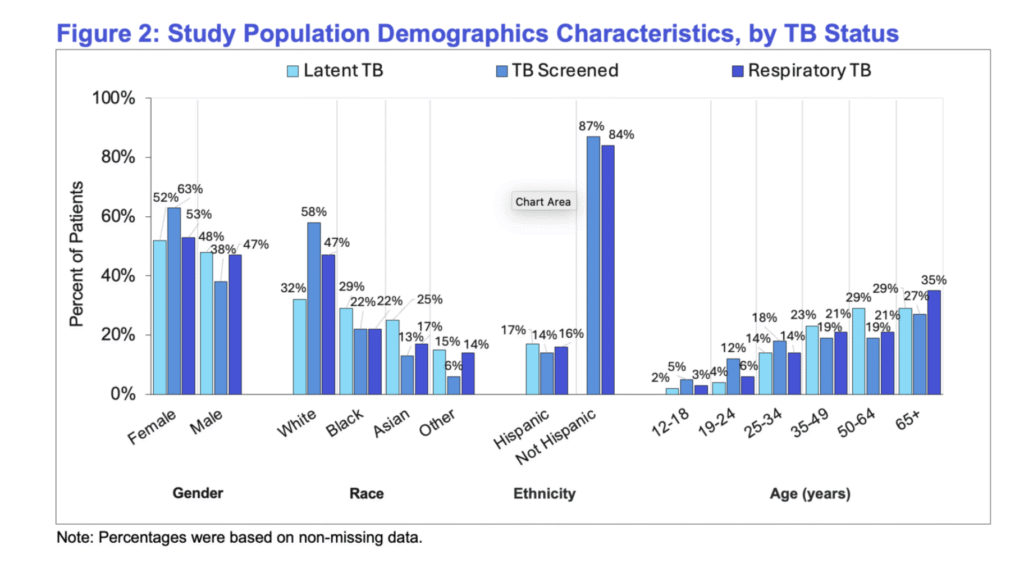
Figure 3. Percentage of Affirmative Responses Across Social Determinants of Health Domains, by TB Status
Why It Matters: Addressing Gaps in TB Prevention
The study emphasizes early detection and screening to better prevent TB reactivation. The differences identified in screening rates indicate that demographic and social factors play a vital role to prevent TB. More targeted interventions can be developed to reduce inequities and improve outcomes if proper identification of populations (with limited access to screening and care) is done.
Looking Ahead
OMNY Health leverages real-world EHR data to better understand patient journeys enhancing preventive care. Future research is aimed to expand the SDoH integration into predictive modeling and public health decision-making. This will be helpful to bridge the gap between data and actionable outcomes.
References
- Centers for Disease Control and Prevention. National Data: Reported Tuberculosis in the United States, 2023. Reported Tuberculosis in the United States, 2023. 2024 Nov 7. Accessed February 10, 2025. https://www.cdc.gov/tb-surveillance-report-2023/summary/national.html
- US Preventive Services Task Force. Screening for Latent Tuberculosis Infection in Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2023;329(17):1487–1494. doi:10.1001/jama.2023.4899C.
© 2025 OMNY Health





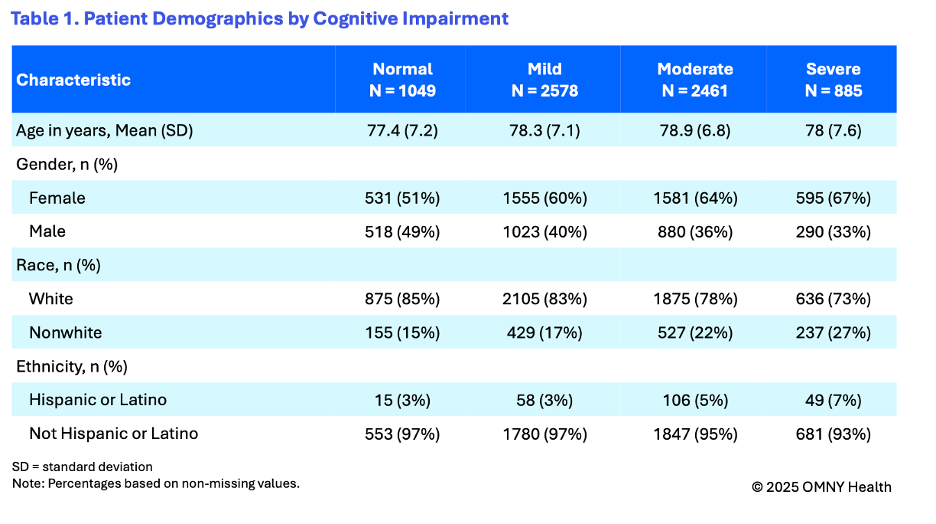
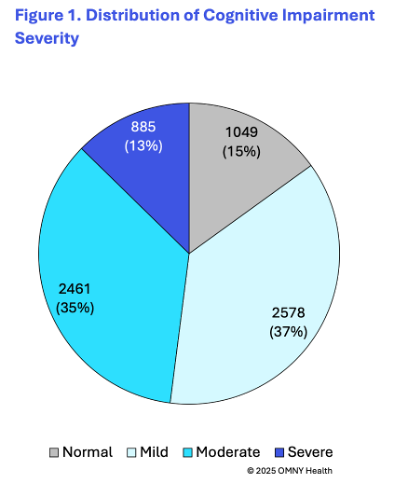 The findings provide insight into the cognitive state of patients as they are first observed within the health system and receiving a diagnosis of Alzheimer’s disease. On average patients were 78 years of age at the time of their first observed diagnosis. Following the first observed diagnosis of Alzheimer’s, patients were categorized into four groups based on their first reported cognitive scores: normal, mild, moderate, and severe.
The findings provide insight into the cognitive state of patients as they are first observed within the health system and receiving a diagnosis of Alzheimer’s disease. On average patients were 78 years of age at the time of their first observed diagnosis. Following the first observed diagnosis of Alzheimer’s, patients were categorized into four groups based on their first reported cognitive scores: normal, mild, moderate, and severe.